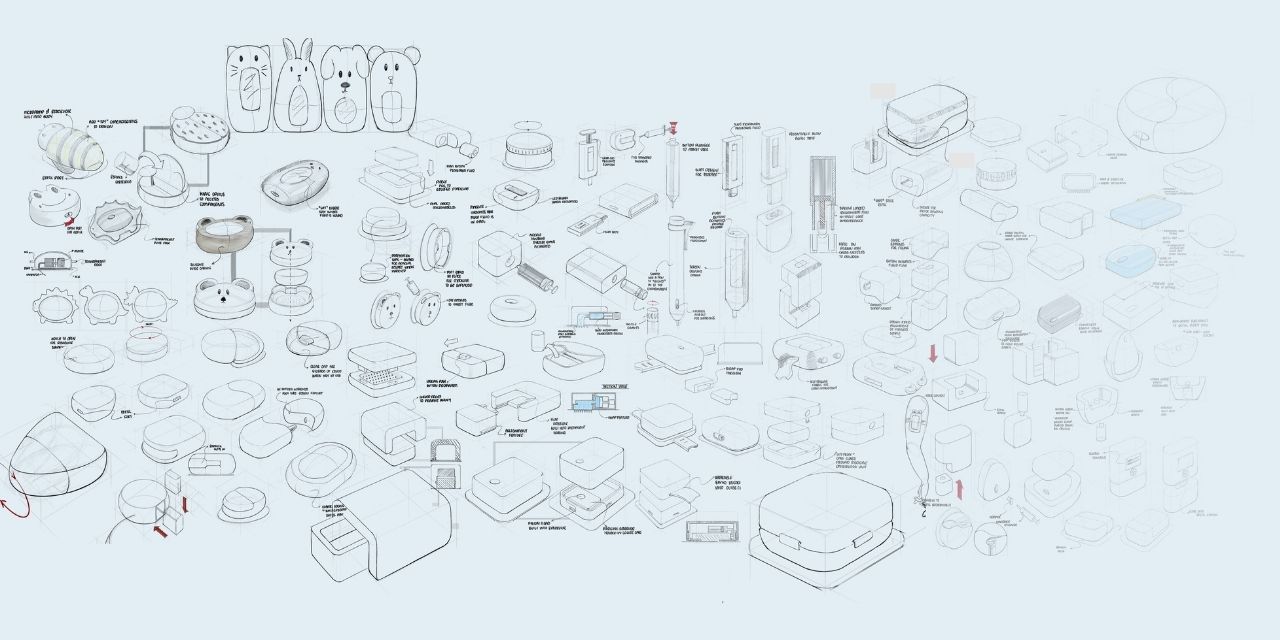
Remedi demonstrates a highly professional and methodical design process, effectively addressing the challenge of improving peadiatric medication adherence through user-focused innovation. The project followed a structured user-centered design approach, beginning with research that identified 95% of children aged 12-18 fail to complete regular medication and antibiotic treatments due to pain, discomfort, and poor time management.
Early user interviews and stakeholder engagement ensured that design decisions were based on real-world needs, rather than assumptions. An iterative development process involved form prototyping, mechanism testing, and refinement based on continual feedback. Despite university capstone constraints of limited budget and timeframes, the project maintained professional standards through systematic validation processes. Finite element analysis (FEA) ensured structural integrity, while design for manufacture (DFM) and design for assembly (DFA) validated commercial viability, and cost analysis confirmed economic feasibility, allowing the project to progress from concept sketches to manufacture-ready CAD models. While the original brief focused on improving medication adherence, Remedi exceeded expectations by simultaneously addressing multiple challenges: eliminating pain through 0.25mm microneedles, ensuring precision via optical sensors and linear actuators, incorporating sustainability through modular design, and creating scalability for varied treatments and patient needs.
The final wearable design integrates advanced technologies into a compact 20x60x45mm form. Key features include an innovative push-valve system within the microneedle array that ensures sterile, fluid-tight delivery, with a snap-on mechanism to enable easy component replacement without complex procedures, replaceable reservoirs and microneedle arrays to reduce waste, LED indicators for user feedback, and ingress protection to prevent contamination. The design process culminated in a functional test bench prototype, validating core mechanisms, while manufacture-ready CAD files demonstrate commercial readiness. This progression from concept to implementable solution showcases a robust and professional design methodology that transforms research insights into tangible healthcare innovation, establishing new benchmarks for medical device development.