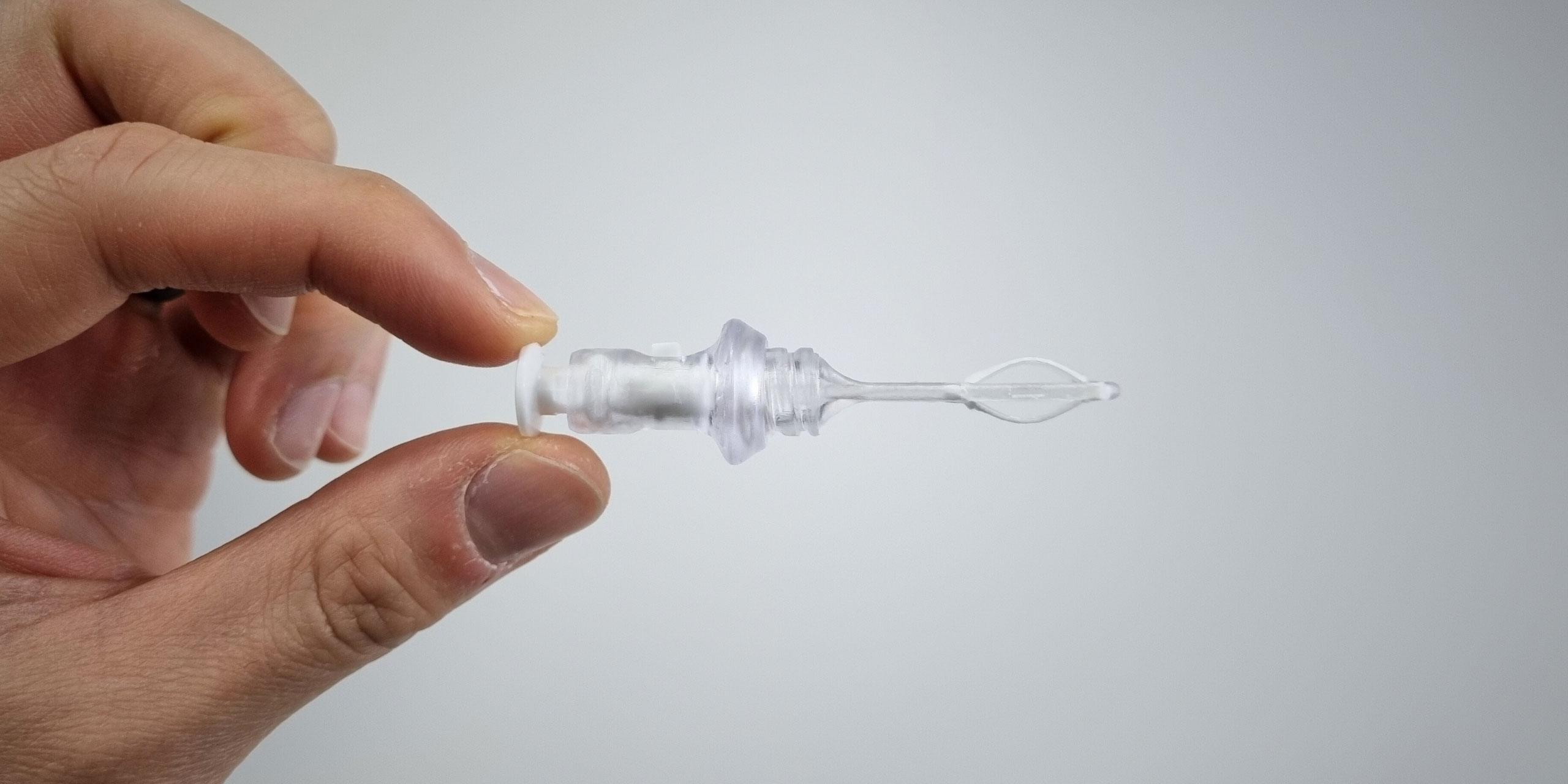
The development of the ABEL Microsampler® followed a systematic medical device design process, beginning with in-depth user research to identify the limitations of existing nasal sampling methods and the unmet needs in the space.
Insights from over 30 healthcare professionals, including ENT specialists, respiratory clinicians, and nurses, were translated into formal design input requirements. These formed the foundation for the device’s development, ensuring alignment with real-world clinical and research needs.
Selection of the ABEL Microsampler®’s collection methodology was driven by a comprehensive literature review and extensive laboratory testing to identify the optimal approach for high-quality nasal fluid sampling. Efficient recovery of proteins and analytes, along with compatibility with modern diagnostic platforms, were identified as critical success factors for the device’s performance.
Bench studies were conducted to evaluate various absorbent materials and collection geometries, with specific focus on analyte preservation and recovery, ensuring samples remain viable for advanced biomarker analysis after collection.
Human factors and usability considerations guided the early concept phase, with particular focus on probe expansion, site-specific targeting, and ergonomic handling. The device was intentionally dimensioned to accommodate a broad range of nasal anatomies while minimising material use.
Iterative prototyping and bench testing were conducted at Diag-Nose Medical’s facility in Victoria, with each design cycle informed by clinician feedback and technical evaluation.
Material selection prioritised patient safety, durability, and biocompatibility, culminating in the selection of suitable medical-grade materials.
A built-in depth limiter was engineered as a critical safety feature to prevent over-insertion and mitigate risk of skull base trauma.
The final design underwent full verification, validation, and compliance testing to meet safety and regulatory standards.
With ARTG registration and FDA listing secured, the ABEL Microsampler® is an innovative device that enables consistent, site-specific nasal fluid collection, revolutionising respiratory research and precision diagnostics.