Navi Medical Technologies engaged a multidisciplinary team of industry experts, biomedical engineers, industry partners, and a senior neonatologist in the development of the Neonav® System. The process began with extensive research in neonatal and paediatric intensive care units across Australia and the USA, gathering first-hand insights from clinical staff about the challenges of reliable and repeatable catheter tip location.
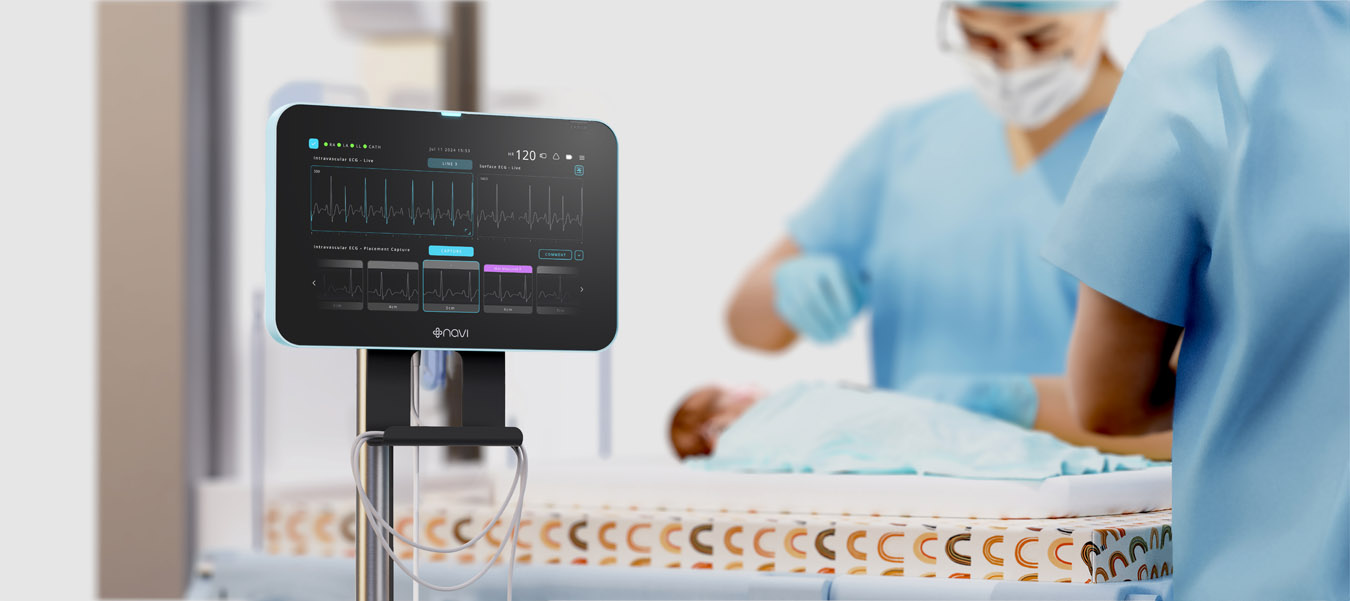
Phase one involved identifying problems and defining user and operational environment needs, highlighting the necessity for real-time feedback during catheter insertion and ongoing surveillance to prevent migration. Navi developed several prototype iterations, incorporating feedback from healthcare professionals. These prototypes were tested in controlled clinical environments on over 100 babies, ensuring they met safety and efficacy standards.
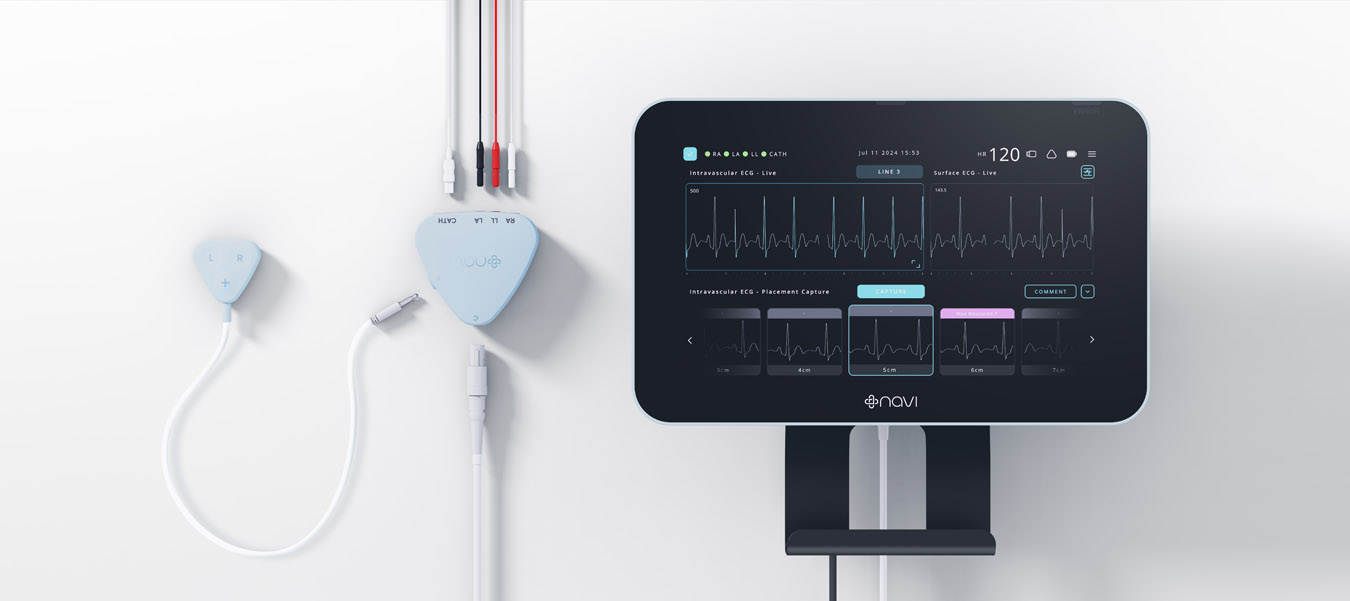
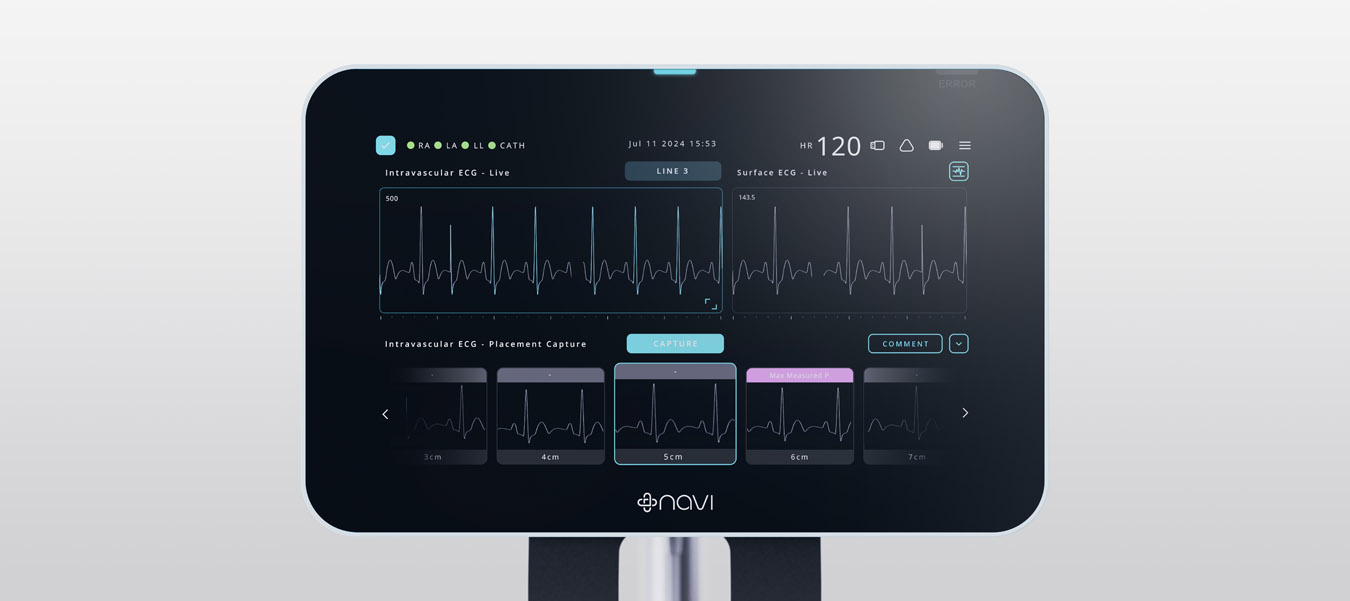
A significant milestone was achieved with FDA Breakthrough Device Designation, validating the system’s approach, where only medical devices that demonstrate a significant improvement over existing solutions for patient healthcare outcomes are granted such a designation from the FDA. The design incorporated Navi’s proprietary software and hardware solutions for accurate ECG signal interpretation and real-time catheter tip location feedback. The team focused on a non-invasive, user-friendly device that integrates easily into existing clinical workflows.
Collaboration with partners like the University of Melbourne, the Royal Women’s Hospital, and Design + Industry (D+I) ensured rigorous testing and refinement. D+I’s design and development processes were compliant with ISO 13485:2016, ensuring the highest safety, reliability, and usability standards in line with regulations for medical devices.
The system underwent extensive clinical trials, including the ”ECHO” study, which confirmed the device’s precision and reliability compared to ultrasound technology. Navi progressed the technology through research trials and test builds, gaining a solid understanding of system use cases, workflows, and users. The clinical evaluation with over 100 newborn participants demonstrated the technology’s accuracy. The final design exceeded the brief by providing real-time feedback during catheter insertion and continuous post-procedure surveillance, reducing the need for confirmatory X-rays and multiple insertion attempts.
The system addresses critical challenges in paediatric and neonatal care, enhancing patient safety, improves clinical efficiency, and reduces healthcare costs.