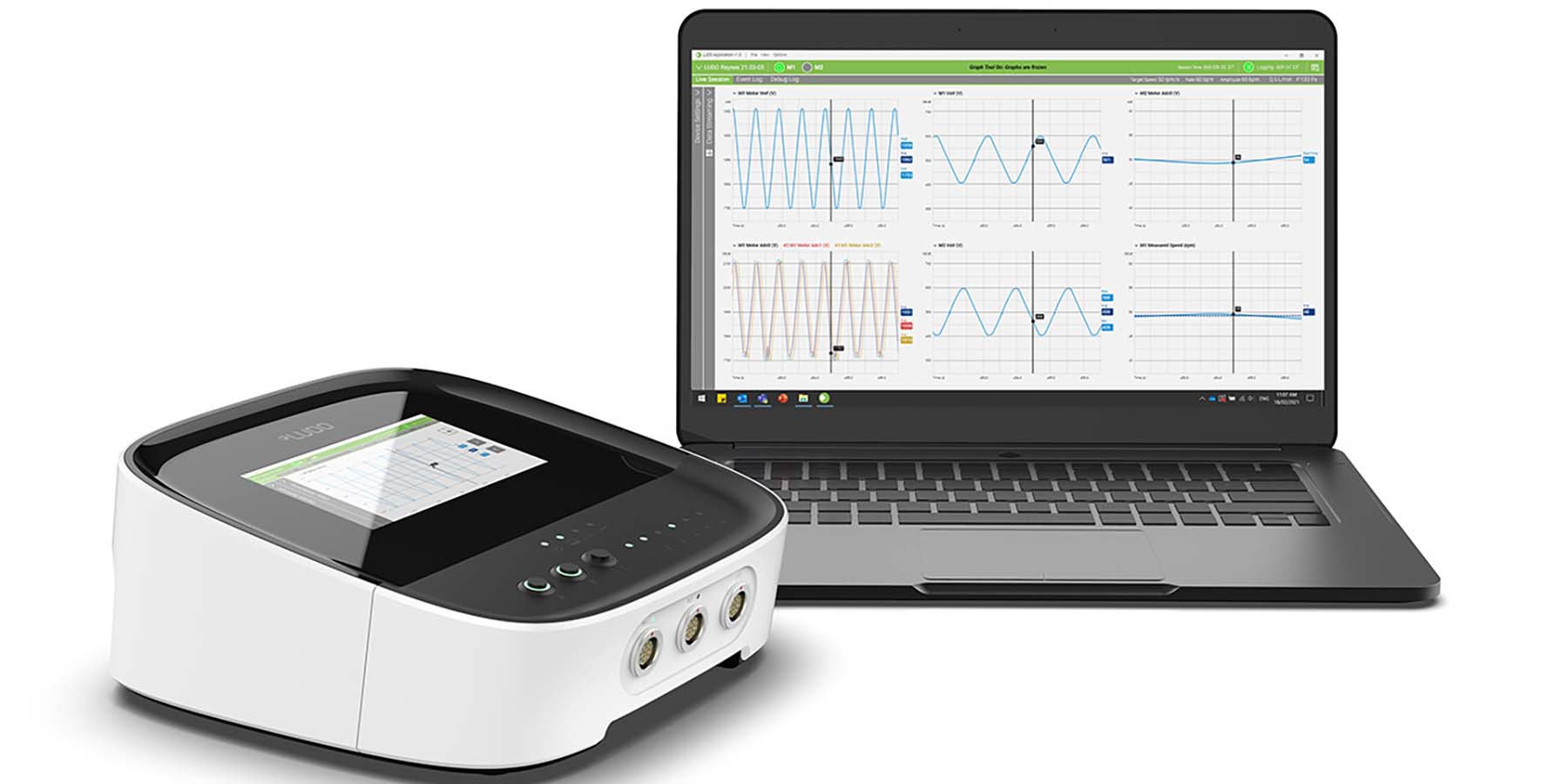
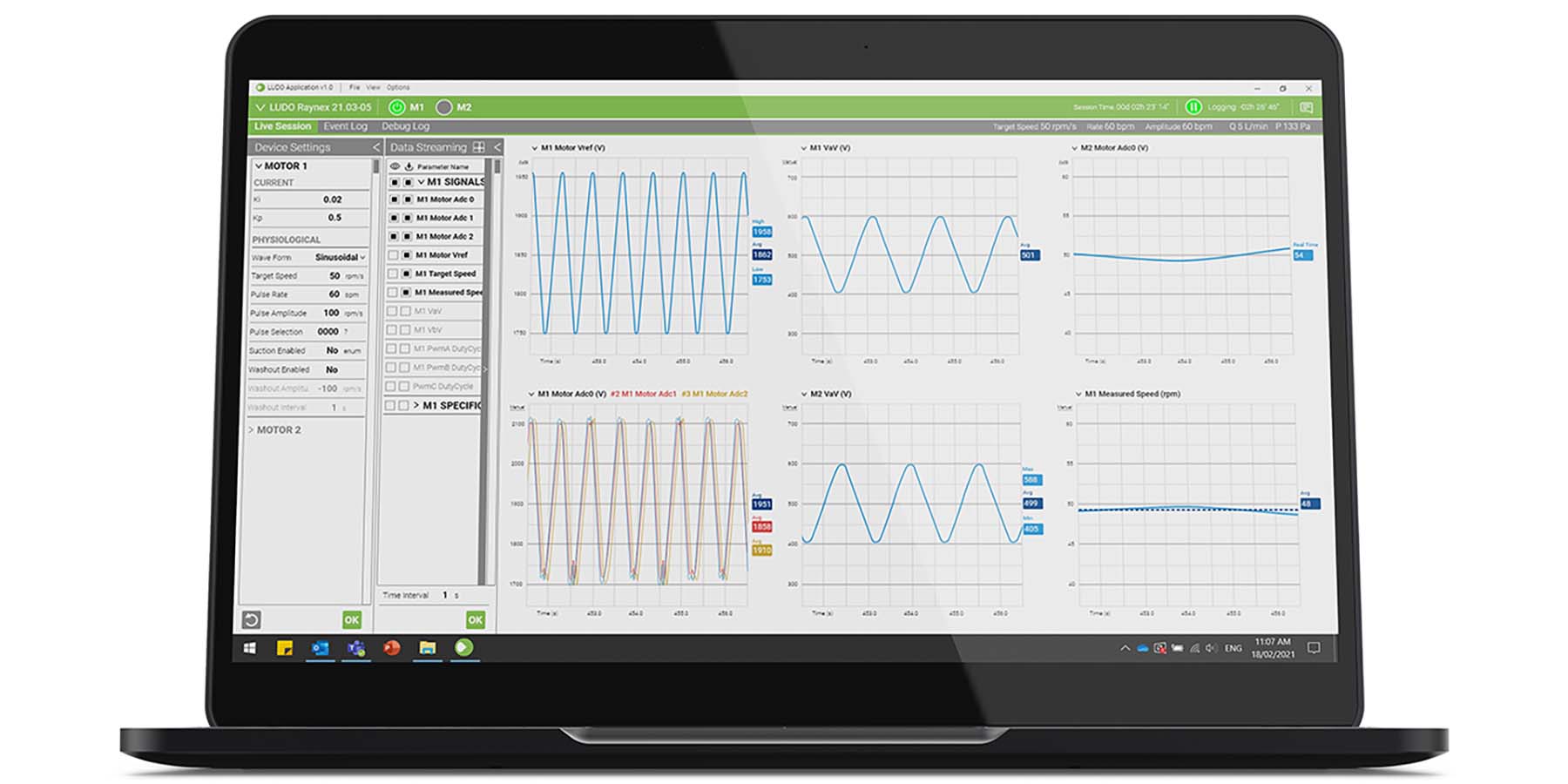
The LUDO system was developed by professional engineers, designers, and scientists to meet the key requirements of Total Artificial Heart development community. It was designed to control a range of pump technologies for any medical device design including rotary and reciprocating devices driven via electric motors, pneumatics or hydraulics, from research programs and early-stage pump refinement to clinical system development. LUDO combines Safety Critical Architecture, proven subsystems and software modules, and a configurable, compact controller box enabling developers greater development flexibility in refining their unique pump.
The controller includes:
- Architecture and components suitable for a future Class III medical device
- Feature sets enabling ease of integration of physiological and motor control algorithms
- Sensor inputs/outputs
- Programmable LED indicators & switches
- Data streaming to the LUDO User Application
The entire system was developed with end-users in mind, with user/industry feedback gathered and assessed as part of the development process.