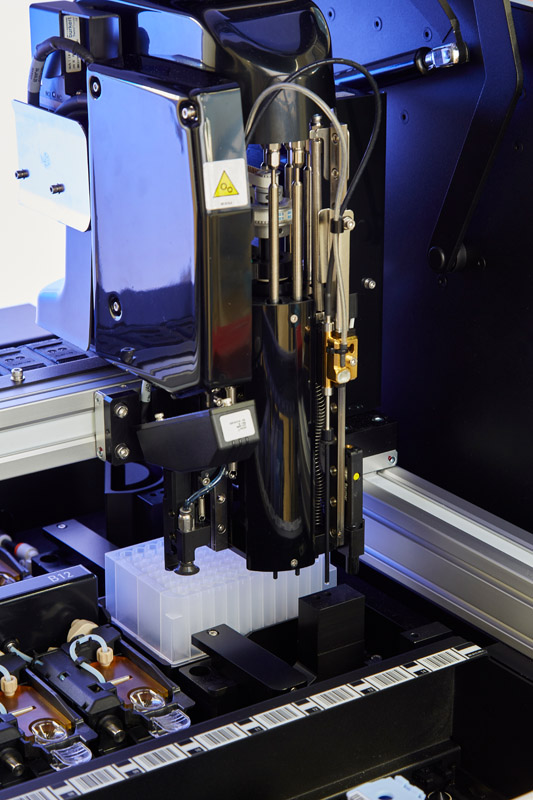
The entire design and development phase was conducted under the rigorous design controls expected of a system that is intended for clinical diagnostic purposes with the required clearances and approvals.
The design phase of the BOND-PRIME program was anchored around extensive Voice of Customer surveys that identified the “pain points” experienced by both laboratory users and pathologists with existing IHC staining systems.
The feedback was used to quantify what level of "breakthrough" would be necessary to create meaningful impact to their operations. For example, it was determined that the diagnostic process would greatly benefit if staining times were reduced from the existing average of 2.5 hours to a 90-minute average.
The development team used iterative ideation and rapid concept development to ascertain which technologies could be adopted with incremental innovation, and which would require “breakthrough” innovation.
Key sub-systems were rapid prototyped to demonstrate feasibility against the design criteria, with incremental iterations necessary to achieve the target performance. Critical-to-quality subsystems were identified, and performance improvements "allocated" to different teams to ensure the finished product remained aligned with the design brief. For example, it took a coordinated effort from individual specialist teams to achieve quality and increased speed: a software team for the robotic scheduler, a mechatronics team for the robotics, and a science team to optimize the staining protocols.
With feasibility of the sub-systems proven, the product design team brought together the complete technical solution in iterative releases. Parallel verification testing was conducted to ensure the detailed integrated system continued to deliver not only the high-level product requirements, but also quality, reliability, and cost targets.